Qvin™, the biotechnology research company that developed the first and only healthcare service that collects menstrual blood samples as an alternative to traditionally collected venous blood draws, has announced FDA clearance for its innovative Q-Pad™ and A1c Test. This clearance allows for the collection of menstrual blood samples, offering an alternative to conventional venous blood draws for millions of American women managing diabetes. This breakthrough marks an opportunity to conveniently monitor their A1c levels, and gives the opportunity to test important biomarkers for the more than 80 million people who menstruate in the U.S.
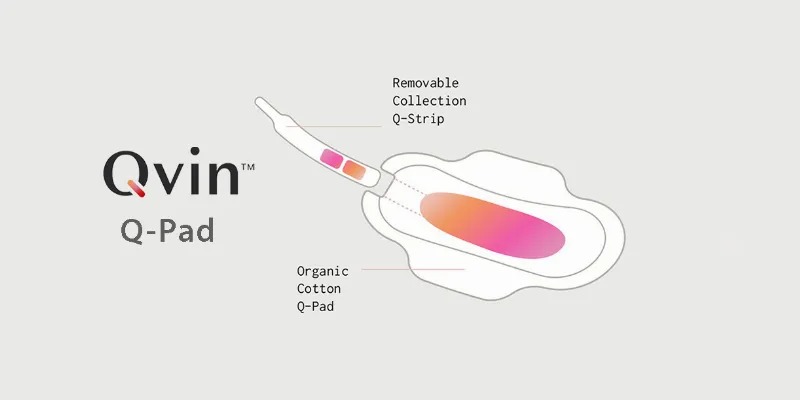
Each Q-Pad includes a removable strip; once the Q-Pad has sufficiently collected a menstrual sample, the removed collection strip is sent to a CLIA-Certified (Clinical Laboratory Improvement Amendments) laboratory for clinical testing. Users receive their results via the free and convenient Qvin app.
Qvin’s research confirms the clinical importance of menstrual blood for multiple vital biomarkers, despite the fact that billions of people worldwide experience monthly menstruation. Menstrual sample collection at home is made possible by the Q-Pad technology, which is revolutionizing diagnostics.
Qvin, in collaboration with researchers at academic institutions such as Stanford University School of Medicine, has published peer-reviewed research validating other biomarkers that can also be monitored. Q-Pad allows individuals to submit specimens directly to the lab and receive reports on key health conditions that often go undiagnosed or misdiagnosed including pre/diabetes, anemia, fertility, perimenopause, endometriosis, and thyroid health.



